
The Role and Importance of CO2 Specification on Transport and Storage Networks
16 April 2025

Citation: IEAGHG, “The Role and Importance of CO2 Specification on Transport and Storage Networks”, 2025-IP01, April 2025, doi.org/10.62849/2025-IP01.
Following a recent publication by Sonke et al,[1] Shell’s Joop van der Steen (JvdS) discussed with IEAGHG’s Keith Burnard (KB) some Shell-led developments focused on the impact of CO2 specification on CO2 transport and storage networks (Cautionary note | Shell Global).
Introduction and context.
Early CCS projects tended to be vertically integrated, where CO2 was captured from a single source and transported to a dedicated geological store. As the deployment of CCS gains pace, there has been an important shift in management of the CCS chain. Part-chain projects, focusing on capture, transport, or dedicated storage, are developing in connection with emerging shared infrastructure within CCS hubs. With the advent of CCS hubs, CO2 is captured from several emitting sources, such as heavy industries and power, and is transported and stored using common infrastructure. Captured CO2 is never pure; there are trace impurities and, unless the CO2 is destined for food use, some of these are left in the CO2 stream. Consequently, transport and storage (T&S) networks need to accept from emitters CO2 that contains various concentrations of a range of impurities.
To understand the types of impurities we need to look at capture. There are four main variants of CO2 capture:
- Post-combustion capture, which captures CO2 directly from flue gases, typically using solvents, sorbents, membranes, or cryogenics;
- Oxyfuel combustion, capture based on burning fuel with an oxygen rich stream instead of air. Resulting in a CO2 rich flue gas.
- Pre-combustion capture, which captures CO2 from fossil fuels before combustion is completed;
- Other industrial sources such as cement industry, gas processing.
Depending on the nature of the base facility and the CO2 capture method deployed, the environment may be oxidising or reducing. Impurities present in the captured CO2 stream will depend on several factors, but mainly:
- The base facility process, its operating conditions and usage profile.
- The conditions and chemical composition of the flue/fuel gas, downstream of the technologies deployed to reduce the emission of non-CO2 components.
- The CO2 capture technology deployed.
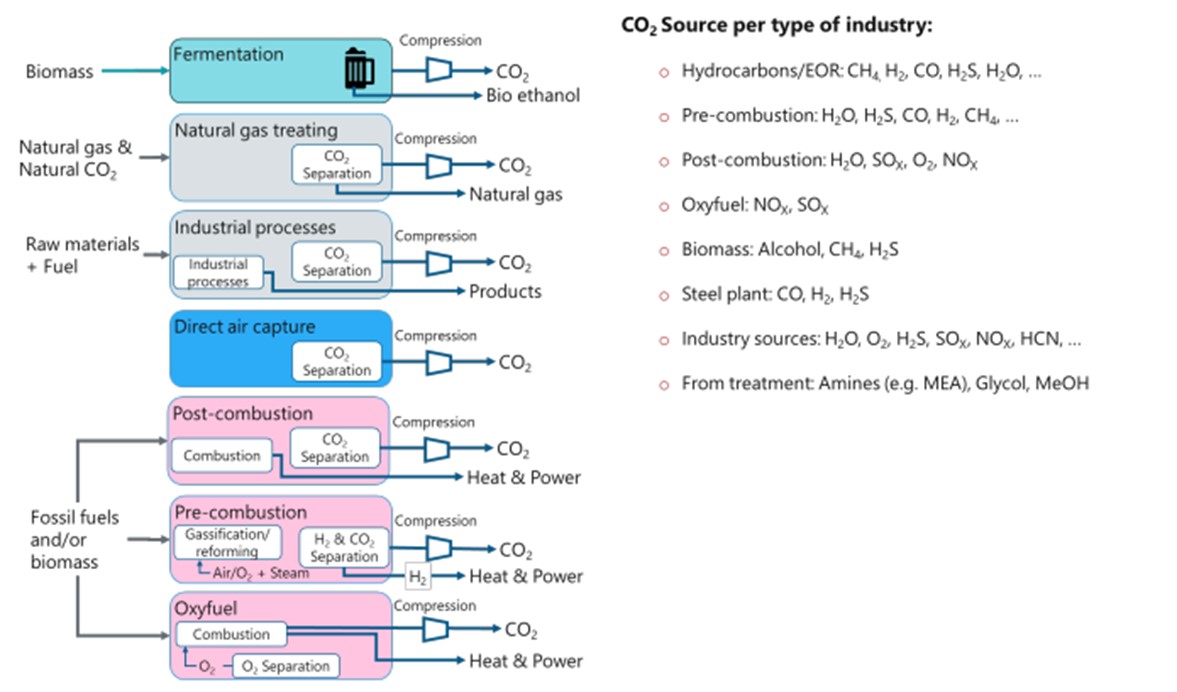
Typical CO2 sources and capture technologies (left) that lead to a typical different range of impurities (right)[2]
At present, there are a number of project-specific specifications for the quality of the CO2 rich gas for CO2 transport, but no unifying methodology for determining what is suitable for a particular network. The gas composition is important owing to its potential to cause damage to the integrity of the transport mode, whether pipeline, ship, barge or truck or train. Combining flows from multiple emitters creates mixtures which can have different effects on the transport and storage infrastructure. Gas conditioning has therefore been elevated from a side to show a crucial aspect of the chain for a successful CCS network.
KB: The mix of impurities in the ‘captured CO2’ stream can have many effects. From your perspective, what are the key effects, and which impurities are the most problematic?
JvdS: Shell’s findings, as discussed in the paper by Sonke et al, shows that impurities in the CO2 stream, including but not limited to SOx, H2S, O2, H2O and NOx, need to be managed to avoid the formation of highly corrosive mixtures, containing sulphuric and/or nitric acid.
For corrosion to occur, the acids need to be formed and need to drop out as a separate phase. Reaction kinetics, and the solubility of these acids, play a key role. Both reaction kinetics and solubility in CO2 stream depend on pressure, temperature and fluid state (vapor, dense, liquid). To make the puzzle even more complex, the PVT behaviour of the CO2 stream (impacting solubility) is impacted by other components e.g. glycols that can be present in the CO2 stream.
KB: Putting acids aside, what other problems can arise from impurities in the captured CO2 stream? How might these problems be exacerbated when combining captured CO2 streams from multiple emitters?
JvdS: Various problems can arise from impurities in the captured CO2 stream, for example:
- Transportation inefficiency in case inert species are present. These inerts will be compressed, transported and stored, which requires energy, hardware and storage space.
- Health, safety, security and environmental (HSSE) impacts to personnel in the event, for example, of venting during maintenance (e.g., H2S, CO, etc.).
- CO2 vapour recovery can lead to contaminants being concentrated in the vapour stream back to ships tanks and at a loading terminal. These can then find their way back into the upstream terminals from which they may not have originated. Potentially exposing staff and equipment to these components.
- Fine dust impacting the injectivity, requiring filtration of particles larger than 1 micron.
- Impact on phase behaviour in pipelines e.g., if components form a separate phase in the pipeline.
KB: What steps can be taken to avoid corrosion? Is it that particular chemical species in the captured CO2 stream must be avoided or that particular conditions along the transport infrastructure must be avoided – or both?
JvdS: Both. The formation of corrosive species and the drop-out of corrosive phases need both to be avoided. The first is a matter of limiting the presence of contaminants, the second is matter of solubility of the corrosive species in the bulk CO2 stream which is governed by the operating conditions in the system. This applies to the entire CCS chain, as temperature and pressure vary in the system and corrosive species formed in one part of the system may drop out in another part of the system. For example, chemical reactions generally happen faster at higher temperatures (in compressors) and at lower temperatures (for example during transport by pipeline) solubility of acids reduces, which may than drop out. For cold liquid CO2 transport solubility is even lower.
Different tactics can be applied, for example:
- Control total acid source atoms, i.e., total S (H2S+ SOx) to control H2SO4 and NOx to control HNO3.
- Control total H-donor (H2O, H2S), as this will limit the acid formation and drop-out.
- Control oxidising agents (O2, NO2), as this will limit acid formation and drop out.
Or a mix of these tactics.
KB: If a particular species in the captured CO2 stream needs to be avoided or reduced in concentration, at what stage along the CO2 value chain would problematic species in the captured CO2 streams need to be addressed – before or after they converge?
JvdS: Finding the optimum, safe specification and best location for producing transport quality CO2 for CCS hubs is subject of current research and project studies. An important factor is the configuration of the specific CCS chain and the types of emitters. Achieving economies of scale by central processing post-co-mingling is not always a given depending on the required specification and the specifics of the emitted streams. Some emitters may already be within the required limits and, with central processing, these streams will be processed unnecessarily. While central processing is considered a potential risk for the gathering system up to the processing plant, it remains to be confirmed by research.
The continuing research will result in guidelines for projects (role of regulators/governments) and will help to guide future projects and support regulators. For example, Shell was a member of a Joint Industry Partnership (JIP) that recently published guidelines for CO2 specifications.2
KB: Would it be practical to produce a specification for the composition of the captured CO2 stream? Or is it more complicated than that?
JvdS: It depends on the mode of transport as this determines the temperature. Depending on their unique balance of emitters and the capture technology(ies) applied, each CCS hub may have a different optimum CO2 specification. The final levels also depend on the wider spectrum of impurities and actual operating conditions (including start-up, shutdown, and other non-steady state operations). A tighter CO2 specification will likely be required for hubs with broader sets of emitters i.e. incompatible components. In a system with a wide range of emitters it will be more difficult to find a common ground on the best strategy to get to operable specification.
KB: What are the remaining questions surrounding the avoidance of corrosion? What gaps remain in our knowledge and what is the way forward? What role is Shell playing in this effort?
JvdS: Understanding of the exact reaction mechanisms and reaction kinetics that lead to corrosive fluid is still improving. Based on modelling and experiments industry is moving to tight specifications, an important step in the testing process is to assure that the selected specification is robust against all operating scenarios. Shell is an active participant in Joint Industry Programmes and Workgroups on this topic and has been forthcoming in sharing experimental insights as the dissemination on critical corrosion threats is essential for the integrity of the CCS infrastructure independent of its operator.
KB: Given the work Shell has done to date, where would you say responsibility lies in cleaning up the captured CO2 stream sufficiently to avoid corrosion – with the capture operator or the transport operator?
JvdS: This is a shared responsibility, as everyone is interested in lowering the overall cost. This implies that they jointly evaluate the expected risks based on capture process employed and the transport infrastructure foreseen. Subsequently, the optimum approach to ensure the non-corrosivity is selected by the transport operator. As the cleaning of the captured CO2 stream will result in waste streams, the logistics and experience of the operators dealing with this need to be considered. It is expected that in most cases the responsibility of meeting the CO2 specification will end up with the emitters, but there may be cases where the polishing of the CO2 stream is best done by the transport operator.
[1] J Sonke, B H Morland, G Moulie and M S Franke, “Corrosion and chemical reactions in impure CO2’, International Journal of Greenhouse Gas Control, 133, 2024.
[2] www.woodplc.com/insights/reports/Industry-Guidelines-for-Setting-the-CO2-Specification-in-CCUS-Chains.
Other articles you might be interested in
Get the latest CCS news and insights
Get essential news and updates from the CCS sector and the IEAGHG by email.
Can't find what you are looking for?
Whatever you would like to know, our dedicated team of experts is here to help you. Just drop us an email and we will get back to you as soon as we can.
Contact Us Now









